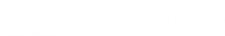Medicines and Healthcare products Regulatory Agency
787 articles
Showing 91-120

Government to align with European specifications on high risk in vitro diagnostic devices to reduce regulatory burden
Thursday, 10 July 2025
The specifications will establish standards for high-risk diagnostic tests while creating consistency with European regulations...

Don’t let the heatwave affect your medicines: Three important tips from the MHRA
Wednesday, 09 July 2025
Essential advice on protecting your medicines during extreme heat and staying safe this summer....

MHRA approves elinzanetant to treat moderate to severe vasomotor symptoms (hot flushes) caused by menopause ?
Monday, 07 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 8 July, become the first regulator in the world to approve elinzanetant (Lyn...

MHRA approves nogapendekin alfa inbakicept to treat adult patients with non muscle invasive bladder cancer??
Friday, 04 July 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 4 July 2025, approved nogapendekin alfa inbakicept (Anktiva) for adults with...

AI Airlock, CERSIs and a new global AI network for health regulators
Thursday, 26 June 2025
Med Tech Regs blog, June 2025: A focus on Software and AI....

If you take a GLP 1 medicine and have been hospitalised by acute pancreatitis, the Yellow Card Biobank wants to hear from you
Thursday, 26 June 2025
GLP-1 medicines are licensed for Type 2 diabetes and weight management, and include the branded products Ozempic, Mounjaro and Wegovy ...

MHRA seizes 7.7 million doses of illegal medicines and removes hundreds of illegal online listings as part of Operation Pangea
Wednesday, 25 June 2025
Operation Pangea brings together health regulators, customs authorities, law enforcement agencies, and private sector partners to tackle the threat po...

MHRA publishes final Business Plan for 2023 2026 Corporate Plan
Wednesday, 25 June 2025
The new Business Plan sets out priorities for 2025–26: Protecting public safety and maintaining public trust; delivering efficient, predictable servic...

UK MHRA leads safe use of AI in healthcare as first country in new global network
Monday, 23 June 2025
The MHRA will help shape international rules for AI in healthcare – speeding up access to safe, effective technologies into the NHS and worldwide....

AI breakthroughs drive expansion of ‘Airlock’ testing programme to support AI powered healthcare innovation
Monday, 23 June 2025
MHRA opens second round of applications to test cutting-edge AI medical technologies following successful pilot phase....
MHRA approves UK’s first anti PD 1 monoclonal antibody for treatment of aggressive form of lung cancer
Friday, 20 June 2025
As with all products, we will keep its safety under close review...
Fast, Expert and Open – how the MHRA is poised to become a global leader in risk proportionate regulation
Wednesday, 18 June 2025
New MHRA CEO puts safety, accelerated access and innovation at the centre of agency’s refreshed strategic direction....
First major overhaul of medical device regulation comes into force across Great Britain
Monday, 16 June 2025
New Post-Market Surveillance (PMS) regulations have taken effect across Great Britain, requiring medical device manufacturers to proactively monitor t...

Chikungunya vaccine (IXCHIQ) temporarily paused in people aged 65 and over as precautionary measure
Monday, 09 June 2025
This is a precautionary measure while the MHRA conducts the safety review. ...

MHRA launches new digital hub in Leeds to drive innovation and regional growth
Friday, 06 June 2025
The new hub will strengthen the MHRA’s work with regional partners and boost the UK’s digital health and life sciences sector....
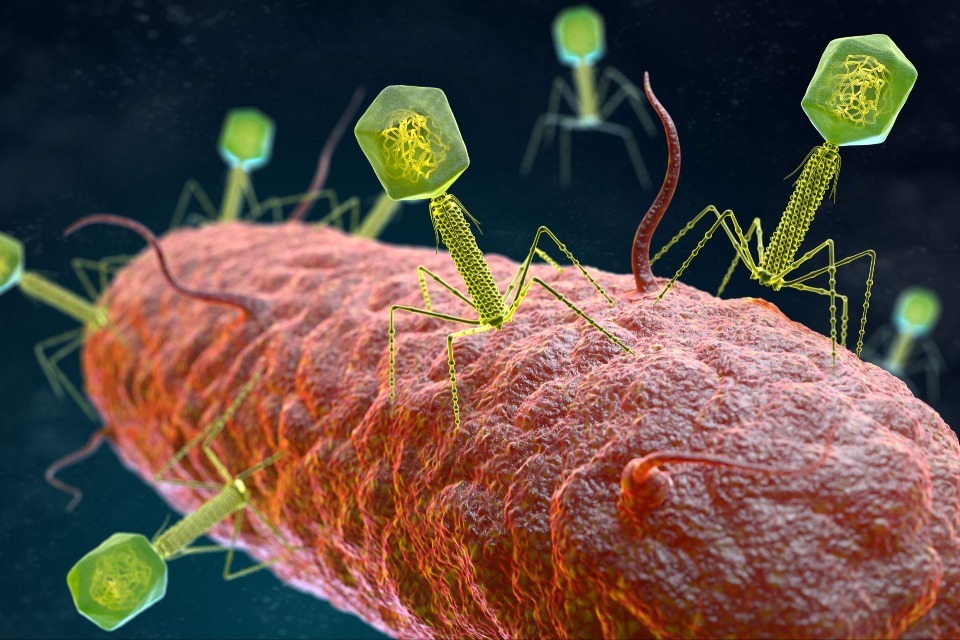
Helping bring phage medicines to UK patients – guidance for industry
Thursday, 05 June 2025
Bacteriophages – viruses that selectively fight bacteria – may offer new hope in fighting infections and tackling antimicrobial resistance....
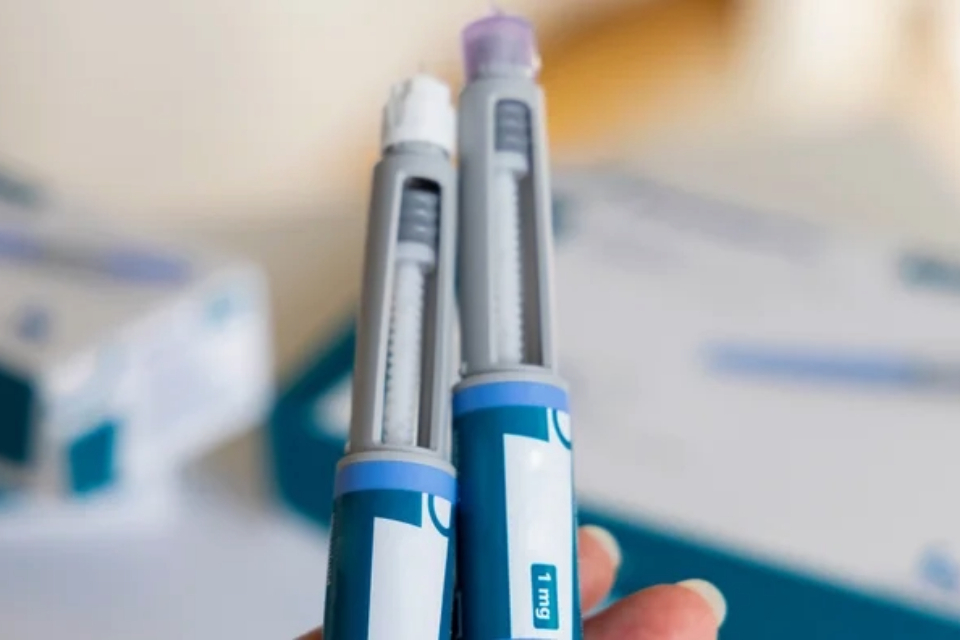
Women on “skinny jabs” must use effective contraception, MHRA urges in latest guidance
Wednesday, 04 June 2025
Anyone who suspects that they’ve had an adverse reaction to their weight loss or diabetes medicine or suspects it is not a genuine product, should rep...

MHRA approves aumolertinib to treat non small cell lung cancer
Monday, 02 June 2025
As with all products, we will keep its safety under close review...

MHRA approves polihexanide to treat acanthamoeba keratitis
Thursday, 22 May 2025
As with any medicine, the MHRA will keep the safety and effectiveness of polihexanide under close review.? ...

First MHRA Board meeting held in Scotland, underlining agency’s commitment to regional health equality and growth
Wednesday, 21 May 2025
The meeting, which took place at the Royal College of Surgeons in Edinburgh, centred on the MHRA’s commitment to delivering the agency’s priorities in...

Building a faster, more effective clinical trials system
Monday, 19 May 2025
By MHRA Chief Executive Lawrence Tallon...

MHRA highlights “remarkable” progress and launches real world data consultation on International Clinical Trials Day
Monday, 19 May 2025
“…the MHRA is once again taking a global lead” says Lord O’Shaughnessy...
MHRA approves guselkumab for Crohn’s disease and ulcerative colitis
Monday, 19 May 2025
As with all products, we will keep its safety under close review...

MHRA approves vaccine to protect against pneumococcal infections such as pneumonia and meningitis
Thursday, 15 May 2025
As with all products, the MHRA will keep its safety under close review....

Meet the women helping ensure that digital mental health technologies are safe, effective and developed considering the needs of the people who use them
Monday, 12 May 2025
Mental health apps are everywhere, offering everything from mood tracking to therapy. But with so many options, how can people tell what these tools a...

MHRA approves first UK treatment for congenital thrombotic thrombocytopenic purpura (cTTP)
Monday, 12 May 2025
As with all products, the MHRA will keep its safety under close review....

MHRA approves world’s first low carbon version of COPD inhaler Trixeo Aerosphere
Monday, 12 May 2025
As with all medicines, the MHRA will continue to monitor the safety and effectiveness of Trixeo Aerosphere...

MHRA approves teprotumumab as the first UK treatment for adults with moderate to severe Thyroid Eye Disease (TED)
Tuesday, 06 May 2025
As with all products, the MHRA will keep its safety under close review....

Vimkunya vaccine approved to prevent disease caused by the chikungunya virus in people 12 years of age and older
Thursday, 01 May 2025
The Medicines and Healthcare products Regulatory Agency (MHRA) has today (1 May 2025) approved a vaccine (Vimkunya) used to prevent disease caused by ...

MHRA authorises cancer treatment variation with an administration time of 3–5 minutes
Wednesday, 30 April 2025
As with all products, the MHRA will keep its safety under close review....