Medicines Healthcare Products Regulatory Agency

MDR number
MDR 065-03/23
Company name
Ferring Pharmaceuticals Limited
Product name
GONAPEPTYL Depot 3.75mg, Powder and solvent for suspension for injection, PL 03194/0085
SNOMED Code
5615111000001102
| Batch number | Expiry date | Pack size | First distributed |
|---|---|---|---|
| T16656K | Mar 2024 | 1 | 19 May 2022 |
| U11271E | Jun 2024 | 1 | 15 Jun 2022 |
| U11271F | Jul 2024 | 1 | 14 Jun 2022 |
| U11386G | Nov 2024 | 1 | 25 Aug 2022 |
| U11570E | Dec 2024 | 1 | 09 Nov 2022 |
| U11571E | Dec 2024 | 1 | 03 Oct 2022 |
| U15102C | May 2025 | 1 | 30 Dec 2022 |
| U15102K | May 2025 | 1 | 13 Feb 2023 |
| U15102L | May 2025 | 1 | 08 Feb 2023 |
Active Pharmaceutical Ingredient: Triptorelin
Brief description of the problem
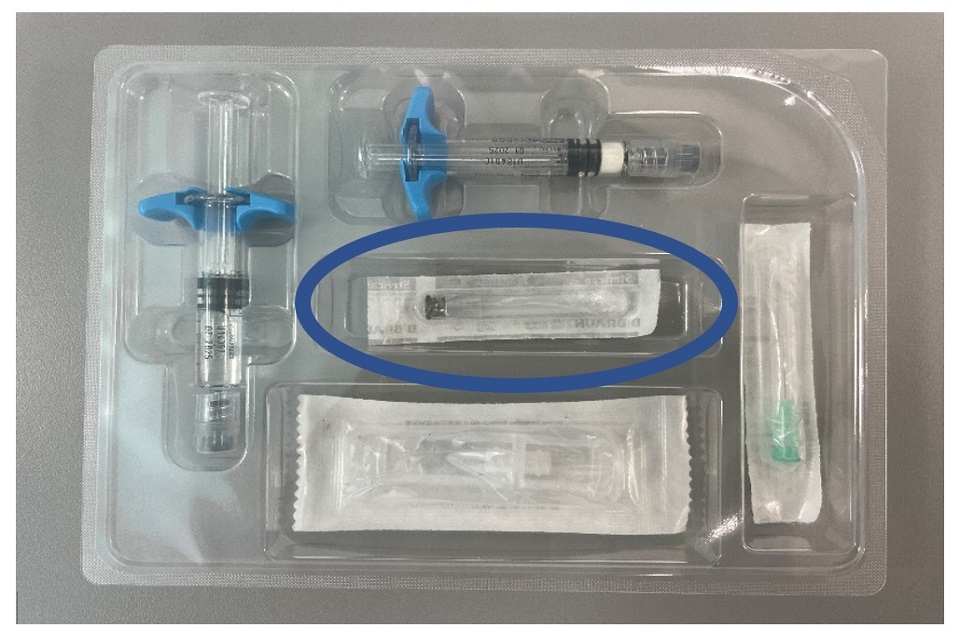
Ferring Pharmaceuticals Limited is recalling certain batches of GONAPEPTYL Depot 3.75mg due to a defect noticed in the seal of the needle wrapping for the CE-marked 30-millimeter (30mm) needle for subcutaneous injection that is supplied with each product pack (see first image in Appendix 1).
Slight damage was detected in some sterile needle blisters, which renders the sealing to be incomplete. A small breach was observed at the peel-away opening of the needle blister, potentially compromising the sterility of the needle. The defect appears as a slight separation between the plastic and paper portions of the needle packaging (see second image in Appendix 1). There is no quality issue with the drug product.
Advice for healthcare professionals
Stop supplying the above batches immediately. Quarantine all remaining stock and return it to your supplier using your suppliers approved process.
Advice for patients
No further action is required by patients as this is a Pharmacy and Wholesaler level recall. This product is administered by healthcare professionals directly. If you have concerns about a medicine you may be using, please contact your healthcare professional.
Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Suspected adverse reactions may also be reported by emailing medical.uk@ferring.com or calling 0800 111 4126.
Further Information
For all medical enquiries, please contact medical information: medical.uk@ferring.com.
For supply queries, please contact customer services: customer.services@ferring.com
Recipients of this Medicines Recall should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Appendix 1: Images of the content of GONAPEPTYL Depot 3.75mg
Image of the 30mm needle packaged within each pack of GONAPEPTYL Depot 3.75mg
Close-up image of a small breach observed at the peel-away needle wrapping for the 30mm needle

